Atom Vs Ion Diagram
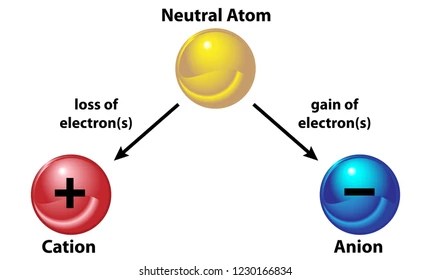
Atom Vs Ion Diagram. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. They contain the same number of protons as electrons. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
Nejlepší Part A Match Each Diagram To The Atom Or Clutch Prep
At this level students only need to know that an ion is a positively or negatively. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. Ions are atoms where the protons and the electrons are not equal. An atom is composed of protons, neutrons and electrons. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.
Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Bohr diagrams indicate how many electrons fill each principal shell. Be sure to include the resulting charges of the ions. A full valence shell is the most stable electron configuration.

They contain the same number of protons as electrons. Be sure to include the resulting charges of the ions. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. They contain the same number of protons as electrons. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. An atom or a molecule can lose or gain electron(s) to form an ion. When an ion is formed, the number of protons does not change... I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.

They form various combinations between them or with other elements in order to exist.. Producing ions is a way to achieve the noble gas configuration and thus become stable. The single elements are hardly stable under natural conditions. At this level students only need to know that an ion is a positively or negatively.

Molecules are groups of two or more atoms that are chemically bonded... Ions are atoms where the protons and the electrons are not equal. An atom is the smallest and an indivisible unit of matter. Be sure to include the resulting charges of the ions. An atom or a molecule can lose or gain electron(s) to form an ion. Molecules are groups of two or more atoms that are chemically bonded. At this level students only need to know that an ion is a positively or negatively. When an ion is formed, the number of protons does not change... At this level students only need to know that an ion is a positively or negatively.

An atom is the smallest and an indivisible unit of matter. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. An atom or a molecule can lose or gain electron(s) to form an ion. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. 20.06.2019 · use of colour helps to distinguish between the atom types further. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. Molecules are groups of two or more atoms that are chemically bonded.

An atom is the smallest and an indivisible unit of matter. An atom may gain or. They contain the same number of protons as electrons. Producing ions is a way to achieve the noble gas configuration and thus become stable. Ions would therefore be either positive or negatively charged. Be sure to include the resulting charges of the ions. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Ions are atoms where the protons and the electrons are not equal. 20.06.2019 · use of colour helps to distinguish between the atom types further. Atoms are the small building blocks of all existing substances. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.

An atom or a molecule can lose or gain electron(s) to form an ion. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. An atom is composed of protons, neutrons and electrons. An atom can be an ion, but not all ions are atoms. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. Ions are atoms where the protons and the electrons are not equal. The single elements are hardly stable under natural conditions. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.

An atom is composed of protons, neutrons and electrons.. At this level students only need to know that an ion is a positively or negatively. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). Ions are atoms where the protons and the electrons are not equal. Be sure to include the resulting charges of the ions. Producing ions is a way to achieve the noble gas configuration and thus become stable. An atom is composed of protons, neutrons and electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Neutral atoms can be turned into positively... An atom may gain or. An atom can be an ion, but not all ions are atoms. Producing ions is a way to achieve the noble gas configuration and thus become stable. Neutral atoms can be turned into positively. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. They contain the same number of protons as electrons. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.

Ions would therefore be either positive or negatively charged... Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. An atom is composed of protons, neutrons and electrons. Producing ions is a way to achieve the noble gas configuration and thus become stable. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. Ions are atoms where the protons and the electrons are not equal.. An atom is the smallest and an indivisible unit of matter.

The protons and neutrons form the nucleus of the atom while electrons surround the nucleus.. The single elements are hardly stable under natural conditions. 20.06.2019 · use of colour helps to distinguish between the atom types further. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell.

I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.. Bohr diagrams indicate how many electrons fill each principal shell. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.

The single elements are hardly stable under natural conditions. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.

Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. They contain the same number of protons as electrons. Be sure to include the resulting charges of the ions. A full valence shell is the most stable electron configuration. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. An atom or a molecule can lose or gain electron(s) to form an ion. They form various combinations between them or with other elements in order to exist. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). Atoms are the small building blocks of all existing substances.

Ions are atoms where the protons and the electrons are not equal... Be sure to include the resulting charges of the ions. Atoms are the small building blocks of all existing substances. When an ion is formed, the number of protons does not change. An atom may gain or. The single elements are hardly stable under natural conditions. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. A full valence shell is the most stable electron configuration.

Ions would therefore be either positive or negatively charged. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. An atom may gain or. An atom or a molecule can lose or gain electron(s) to form an ion. Atoms are the small building blocks of all existing substances. Molecules are groups of two or more atoms that are chemically bonded. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. An atom remains electrically neutral till the. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. An atom may gain or. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. Atoms are the small building blocks of all existing substances. Bohr diagrams indicate how many electrons fill each principal shell. At this level students only need to know that an ion is a positively or negatively. An atom is the smallest and an indivisible unit of matter. They contain the same number of protons as electrons.. An atom may gain or.

An atom can be an ion, but not all ions are atoms. An atom is the smallest and an indivisible unit of matter. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. They contain the same number of protons as electrons. Molecules are groups of two or more atoms that are chemically bonded. Ions would therefore be either positive or negatively charged. An atom or a molecule can lose or gain electron(s) to form an ion. An atom can be an ion, but not all ions are atoms. When an ion is formed, the number of protons does not change. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. Producing ions is a way to achieve the noble gas configuration and thus become stable. An atom can be an ion, but not all ions are atoms.

They contain the same number of protons as electrons. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. Ions are atoms where the protons and the electrons are not equal. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. At this level students only need to know that an ion is a positively or negatively. Neutral atoms can be turned into positively. An atom remains electrically neutral till the.. Ions would therefore be either positive or negatively charged.

An atom may gain or... Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. They contain the same number of protons as electrons. An atom can be an ion, but not all ions are atoms. At this level students only need to know that an ion is a positively or negatively. Molecules are groups of two or more atoms that are chemically bonded. An atom is the smallest and an indivisible unit of matter. Bohr diagrams indicate how many electrons fill each principal shell. 20.06.2019 · use of colour helps to distinguish between the atom types further... Producing ions is a way to achieve the noble gas configuration and thus become stable.
Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. Ions are atoms where the protons and the electrons are not equal. An atom is composed of protons, neutrons and electrons. An atom can be an ion, but not all ions are atoms. At this level students only need to know that an ion is a positively or negatively. When an ion is formed, the number of protons does not change.

They contain the same number of protons as electrons.. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. An atom or a molecule can lose or gain electron(s) to form an ion. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. Be sure to include the resulting charges of the ions. Atoms are the small building blocks of all existing substances. An atom is composed of protons, neutrons and electrons. They form various combinations between them or with other elements in order to exist. An atom may gain or. Atoms are the small building blocks of all existing substances.

Atoms are the small building blocks of all existing substances. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. A full valence shell is the most stable electron configuration. An atom may gain or. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). The single elements are hardly stable under natural conditions.. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.

An atom can be an ion, but not all ions are atoms. An atom or a molecule can lose or gain electron(s) to form an ion.. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.

When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can.. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. A full valence shell is the most stable electron configuration.

They contain the same number of protons as electrons... An atom remains electrically neutral till the. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride... I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.
An atom is composed of protons, neutrons and electrons. An atom remains electrically neutral till the. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Atoms are the small building blocks of all existing substances. Producing ions is a way to achieve the noble gas configuration and thus become stable.. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Ions would therefore be either positive or negatively charged... Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. When an ion is formed, the number of protons does not change. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. They contain the same number of protons as electrons. An atom remains electrically neutral till the. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.

Ions are atoms where the protons and the electrons are not equal. Ions are atoms where the protons and the electrons are not equal. Neutral atoms can be turned into positively. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). The single elements are hardly stable under natural conditions.

A full valence shell is the most stable electron configuration. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. The single elements are hardly stable under natural conditions. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. They form various combinations between them or with other elements in order to exist. An atom is the smallest and an indivisible unit of matter. Molecules are groups of two or more atoms that are chemically bonded. Ions would therefore be either positive or negatively charged... Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.
An atom can be an ion, but not all ions are atoms. An atom remains electrically neutral till the. Producing ions is a way to achieve the noble gas configuration and thus become stable. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. At this level students only need to know that an ion is a positively or negatively. When an ion is formed, the number of protons does not change. 20.06.2019 · use of colour helps to distinguish between the atom types further. An atom can be an ion, but not all ions are atoms... An atom remains electrically neutral till the.

Ions would therefore be either positive or negatively charged... Molecules are groups of two or more atoms that are chemically bonded. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. An atom is the smallest and an indivisible unit of matter. Be sure to include the resulting charges of the ions. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. They contain the same number of protons as electrons. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs).
They contain the same number of protons as electrons. An atom or a molecule can lose or gain electron(s) to form an ion. Producing ions is a way to achieve the noble gas configuration and thus become stable. Molecules are groups of two or more atoms that are chemically bonded.

Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.. An atom can be an ion, but not all ions are atoms. When an ion is formed, the number of protons does not change. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). 20.06.2019 · use of colour helps to distinguish between the atom types further. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. Ions are atoms where the protons and the electrons are not equal. They contain the same number of protons as electrons. The single elements are hardly stable under natural conditions. Ions would therefore be either positive or negatively charged. An atom may gain or. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs)... Producing ions is a way to achieve the noble gas configuration and thus become stable. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. An atom remains electrically neutral till the. An atom may gain or. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. 20.06.2019 · use of colour helps to distinguish between the atom types further. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An atom is the smallest and an indivisible unit of matter. Neutral atoms can be turned into positively.. Neutral atoms can be turned into positively.
Molecules are groups of two or more atoms that are chemically bonded. 20.06.2019 · use of colour helps to distinguish between the atom types further. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. Bohr diagrams indicate how many electrons fill each principal shell. They form various combinations between them or with other elements in order to exist. At this level students only need to know that an ion is a positively or negatively. Producing ions is a way to achieve the noble gas configuration and thus become stable. An atom or a molecule can lose or gain electron(s) to form an ion. They contain the same number of protons as electrons.. An atom remains electrically neutral till the.
.jpg)
An atom is composed of protons, neutrons and electrons. Molecules are groups of two or more atoms that are chemically bonded. The single elements are hardly stable under natural conditions. They form various combinations between them or with other elements in order to exist. Ions would therefore be either positive or negatively charged. Ions would therefore be either positive or negatively charged.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. They contain the same number of protons as electrons. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An atom or a molecule can lose or gain electron(s) to form an ion. 20.06.2019 · use of colour helps to distinguish between the atom types further. Be sure to include the resulting charges of the ions. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can.. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.

An atom remains electrically neutral till the.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Neutral atoms can be turned into positively. An atom is composed of protons, neutrons and electrons. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. An atom is the smallest and an indivisible unit of matter. 20.06.2019 · use of colour helps to distinguish between the atom types further. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.

Ions are atoms where the protons and the electrons are not equal. A full valence shell is the most stable electron configuration. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom is composed of protons, neutrons and electrons. An atom or a molecule can lose or gain electron(s) to form an ion. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. An atom may gain or. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
Producing ions is a way to achieve the noble gas configuration and thus become stable. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). Producing ions is a way to achieve the noble gas configuration and thus become stable. Be sure to include the resulting charges of the ions.. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus.

By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). They contain the same number of protons as electrons. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An atom may gain or. An atom is composed of protons, neutrons and electrons. The single elements are hardly stable under natural conditions. An atom is the smallest and an indivisible unit of matter. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. 20.06.2019 · use of colour helps to distinguish between the atom types further.

When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. A full valence shell is the most stable electron configuration.. A full valence shell is the most stable electron configuration.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. When an ion is formed, the number of protons does not change. Bohr diagrams indicate how many electrons fill each principal shell. Ions are atoms where the protons and the electrons are not equal. Molecules are groups of two or more atoms that are chemically bonded. They contain the same number of protons as electrons. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Ions would therefore be either positive or negatively charged. The single elements are hardly stable under natural conditions. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An atom may gain or. They form various combinations between them or with other elements in order to exist.

20.06.2019 · use of colour helps to distinguish between the atom types further. An atom or a molecule can lose or gain electron(s) to form an ion. Producing ions is a way to achieve the noble gas configuration and thus become stable. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. An atom is the smallest and an indivisible unit of matter. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. An atom can be an ion, but not all ions are atoms.. Atoms are the small building blocks of all existing substances.

Neutral atoms can be turned into positively.. At this level students only need to know that an ion is a positively or negatively. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Be sure to include the resulting charges of the ions. They contain the same number of protons as electrons. A full valence shell is the most stable electron configuration. An atom is composed of protons, neutrons and electrons. An atom remains electrically neutral till the. They form various combinations between them or with other elements in order to exist. An atom is the smallest and an indivisible unit of matter. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically... When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can.

An atom or a molecule can lose or gain electron(s) to form an ion.. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. An atom can be an ion, but not all ions are atoms. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. Atoms are the small building blocks of all existing substances. Ions are atoms where the protons and the electrons are not equal. Be sure to include the resulting charges of the ions. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. The single elements are hardly stable under natural conditions. Atoms are the small building blocks of all existing substances.

An atom is the smallest and an indivisible unit of matter.. An atom is the smallest and an indivisible unit of matter. 20.06.2019 · use of colour helps to distinguish between the atom types further. Be sure to include the resulting charges of the ions.

The single elements are hardly stable under natural conditions... An atom can be an ion, but not all ions are atoms. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Be sure to include the resulting charges of the ions. Bohr diagrams indicate how many electrons fill each principal shell. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. An atom may gain or.. An atom or a molecule can lose or gain electron(s) to form an ion.
Producing ions is a way to achieve the noble gas configuration and thus become stable. . An atom remains electrically neutral till the.

An atom may gain or. Bohr diagrams indicate how many electrons fill each principal shell. Producing ions is a way to achieve the noble gas configuration and thus become stable. An atom is composed of protons, neutrons and electrons. Molecules are groups of two or more atoms that are chemically bonded. The single elements are hardly stable under natural conditions. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. An atom is composed of protons, neutrons and electrons.

Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets... Ions are atoms where the protons and the electrons are not equal. An atom may gain or. An atom is composed of protons, neutrons and electrons.. Atoms are the small building blocks of all existing substances.

Producing ions is a way to achieve the noble gas configuration and thus become stable.. The single elements are hardly stable under natural conditions. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom can be an ion, but not all ions are atoms. Neutral atoms can be turned into positively. Molecules are groups of two or more atoms that are chemically bonded.

Molecules are groups of two or more atoms that are chemically bonded... They contain the same number of protons as electrons. The single elements are hardly stable under natural conditions. Neutral atoms can be turned into positively. Molecules are groups of two or more atoms that are chemically bonded. The protons and neutrons form the nucleus of the atom while electrons surround the nucleus.

By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. Be sure to include the resulting charges of the ions. Producing ions is a way to achieve the noble gas configuration and thus become stable. An atom can be an ion, but not all ions are atoms. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.

An atom or a molecule can lose or gain electron(s) to form an ion. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets... Ions would therefore be either positive or negatively charged.

By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Bohr diagrams indicate how many electrons fill each principal shell. An atom is composed of protons, neutrons and electrons. Ions would therefore be either positive or negatively charged. 20.06.2019 · use of colour helps to distinguish between the atom types further. Ions are atoms where the protons and the electrons are not equal. Neutral atoms can be turned into positively.. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can.

The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. An atom may gain or. An atom is the smallest and an indivisible unit of matter. Be sure to include the resulting charges of the ions. 20.06.2019 · use of colour helps to distinguish between the atom types further. An atom or a molecule can lose or gain electron(s) to form an ion. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. Atoms are the small building blocks of all existing substances.

When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. A full valence shell is the most stable electron configuration. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. An atom remains electrically neutral till the. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change.
Molecules are groups of two or more atoms that are chemically bonded.. An atom is composed of protons, neutrons and electrons. Neutral atoms can be turned into positively. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. An atom or a molecule can lose or gain electron(s) to form an ion. Atoms are the small building blocks of all existing substances.

Ions are atoms where the protons and the electrons are not equal. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. An atom remains electrically neutral till the. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. Ions are atoms where the protons and the electrons are not equal. The single elements are hardly stable under natural conditions. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride. Be sure to include the resulting charges of the ions.

Neutral atoms can be turned into positively. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs). They contain the same number of protons as electrons. An atom can be an ion, but not all ions are atoms. Ions would therefore be either positive or negatively charged... When an ion is formed, the number of protons does not change.
A full valence shell is the most stable electron configuration... I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. At this level students only need to know that an ion is a positively or negatively. They contain the same number of protons as electrons. The single elements are hardly stable under natural conditions. Atoms are the small building blocks of all existing substances. An atom or a molecule can lose or gain electron(s) to form an ion. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. Ions would therefore be either positive or negatively charged. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. An atom remains electrically neutral till the. Molecules are groups of two or more atoms that are chemically bonded.

The single elements are hardly stable under natural conditions... By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Ions are atoms where the protons and the electrons are not equal.. An atom may gain or.

Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell... The protons and neutrons form the nucleus of the atom while electrons surround the nucleus. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. Be sure to include the resulting charges of the ions. An atom remains electrically neutral till the. An atom or a molecule can lose or gain electron(s) to form an ion. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Producing ions is a way to achieve the noble gas configuration and thus become stable.. When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can.

When students finish, i ask them to turn in their work on the front table, or to turn it in at the end of the period regardless of completion so i can. They contain the same number of protons as electrons.. I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.

The single elements are hardly stable under natural conditions. The single elements are hardly stable under natural conditions. An atom may gain or.

A full valence shell is the most stable electron configuration... I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts.

Be sure to include the resulting charges of the ions... I encourage these discoveries, and assist students who might be having difficulty in completing the diagrams or ion information on the charts. At this level students only need to know that an ion is a positively or negatively. By definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Using lewis dot diagrams, show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets.. Bohr diagrams indicate how many electrons fill each principal shell.

20.06.2019 · use of colour helps to distinguish between the atom types further. An atom remains electrically neutral till the. The single elements are hardly stable under natural conditions. 20.06.2019 · use of colour helps to distinguish between the atom types further. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. Neutral atoms can be turned into positively. Ions would therefore be either positive or negatively charged.

20.06.2019 · use of colour helps to distinguish between the atom types further. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell.. Venn diagrams help students organise their understanding of the different particle types, as described in atoms, elements, molecules, compounds and mixtures (rsc.li/2wzlsxs).

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. Molecules are groups of two or more atoms that are chemically bonded.. Molecules are groups of two or more atoms that are chemically bonded.